Introduction: The Age of Advanced Phage Research
Leading research in 2024 has better explained the intricate host-virus relationship between bacteriophages and their bacterial hosts, showing how these small bacterial predators make use of auxiliary metabolic genes to influence host metabolism. A team from the University of Kaiserslautern-Landau, in cooperation with its international team, presents this breakthrough—a furtherance of our knowledge with regard to phage biology and one that could signal a sea change in attempts to combat antibiotic-resistant bacteria.
Phages and their ecological importance
Bacteriophages, or phages, are viruses infecting bacteria. They are the important regulators of microbial populations, particularly in water bodies. Such organisms take important roles in nutrient cycling and maintenance of microbial diversity on a global scale. Infection of bacteria by phages includes not only the utilization of bacterial machinery for replication but also such ecological processes as nutrient recycling and microbial evolution via horizontal gene transfer. Such effects emphasize the need for phage studies beyond their therapeutic applications.
Auxiliary Metabolic Genes Reprogram the Bacterial Hosts
One of the most striking observations that can be pointed out from the newly conducted studies is the identification and functionality of AMGs in phage genomes. Phages acquire these genes from their bacterial hosts, not for their replication but to reprogram host metabolic processes during infection. In other words, the presence of AMGs would allow them to “reprogram” their bacterial hosts in a way that optimally enhances the metabolic flux of the bacteria toward the production of new virions.
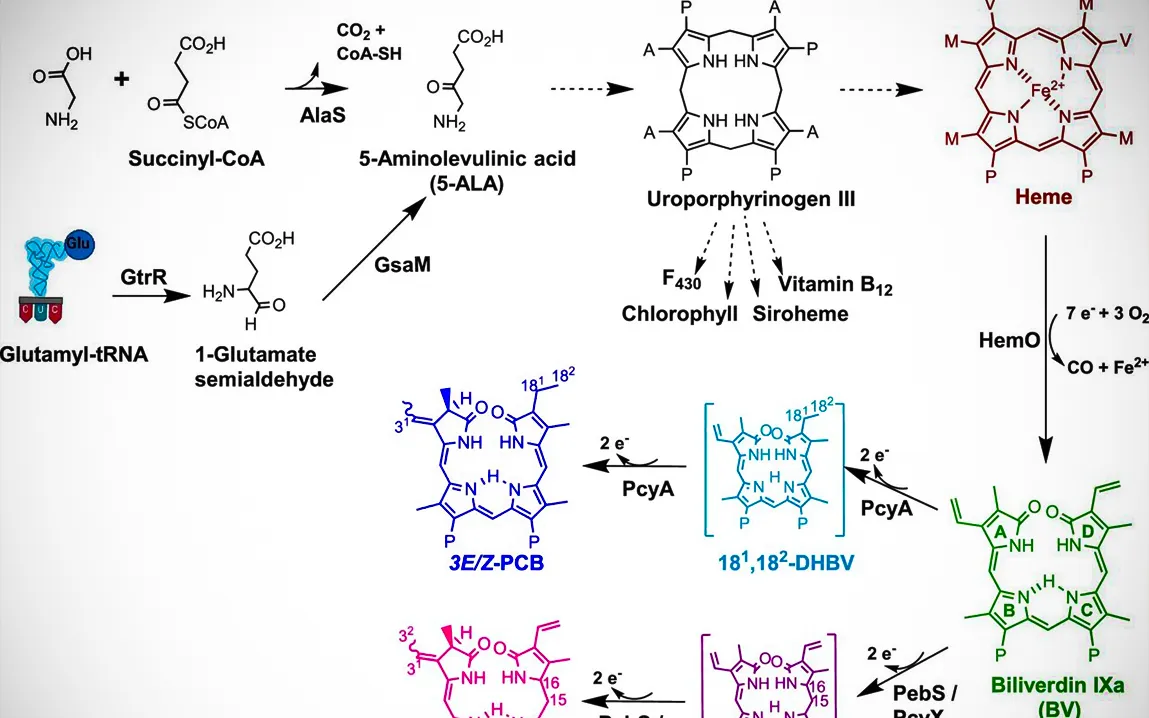
The new research now suggests that these genes are far more pervasive and versatile than previously known. By mapping genetic material from water-related settings, researchers have been able to chart these AMGs, underscoring their functions in some of the most basic metabolic processes, such as the biosynthesis of tetrapyrroles vital in energy production and photosynthetic processes.
Shemin Pathway Gene Discovery: An Evolutionary Perspective
The most salient discovery from this work is the identification of a novel AMG that participates in the Shemin pathway, a metabolic pathway hitherto considered to be the preserve of higher organisms, birds, and mammals, and very few bacteria. Given the efficiency of tetrapyrrole production via the Shemin pathway using just one enzyme, such a pathway would confer on phages an evolutionary advantage. This finding not only shows how phages hijack and optimize bacterial genes for their own benefit but also develops the concept of microbial evolution.
Global Collaboration Efforts: The Role of Bioinformatics
It consisted of an international team of members from Israel, the Netherlands, and Germany. The team has amassed enormous amounts of genetic data obtained from aquatic habitats, such as oceans and lakes, using advanced bioinformatics tools along with environmental sampling. This approach hints toward modern microbiological research, which is essentially collaborative and uses big data for studying organisms such as phages at a microscopic scale.
Probably one of the most striking aspects of this work will be to employ the dataset from the Tara Oceans and Tara Polar Circle expeditions—an enormous repository of genetic material of viruses and bacteria from the different marine environments. This kind of curation and analysis of datasets allows the research team to establish the exhaustive catalog of AMGs, hence identifying the way in which these genes influenced global biogeochemical cycles and microbial ecology.
Journalistic Analysis: Implications for Medicine and Ecology
The implications from a journalistic viewpoint are huge. If phage AMGs are underlined, then one will realize that bacteriophage therapy, much advanced as an alternative to antibiotic treatment, is going to be more specific and efficient. In that scenario, by utilizing the normal capability of phages to change bacterial metabolism, one could develop treatments using altered phages to fight antibiotic-resistant infections or, for that matter, manage microbial populations either in agriculture or the environment.
This also has ecological dimensions: phages already play a vital role in nutrient cycling in aquatic environments, and their potential to regulate bacterial activity via AMGs has the potential to alter carbon and nitrogen cycling on long-term timescales. This ascribes another exciting dimension to phage biology beyond the use of their therapeutic applications but also as ecological engineers in Earth biogeochemical cycling processes.
Future Directions: Furthering the Phage Genetic Catalog
These ongoing efforts to standardize and curate AMG sequences set the trajectory for more findings. Greater elaboration of new AMGs and their ecological functions will emerge from this extended genetic catalog. These will not only refine our concept of phage-host dynamics but also enable further biotechnical applications, ranging from agriculture to medicine.
Besides that, this extension of phage AMGs knowledge would probably be one step toward bioengineering ideas that may open the way to manipulate those genes in order to create synthetic phages that would be industrially and environmentally used. This kind of development would illuminate the scope and promise of phages in being utilized either as an agent of disease or an ecological corrective.
Conclusion: A Promising Future for Phage Research
The discovery of the Shemin pathway gene marks an important point in time where phage-host interactions take on a completely new turn. As the scientific world continues to unravel the genetic arsenal that phages possess, so does the surge of their applications in medicine, agriculture, and environmental science. This milestone in 2024 adds not only to scientific knowledge but also to new perspectives regarding the most daunting health and ecological problems humankind is faced with today.
Most importantly, it remains one of the most exciting and fast-evolving fields; indeed, as seen with the CRISPR/Cas system, it holds the future possibility of Nobel Prize-winning discoveries. Interdisciplinary collaboration and the usage of a global dataset testify to the scientific community’s zest for harnessing the power of these viral entities for the good of mankind and the planet.