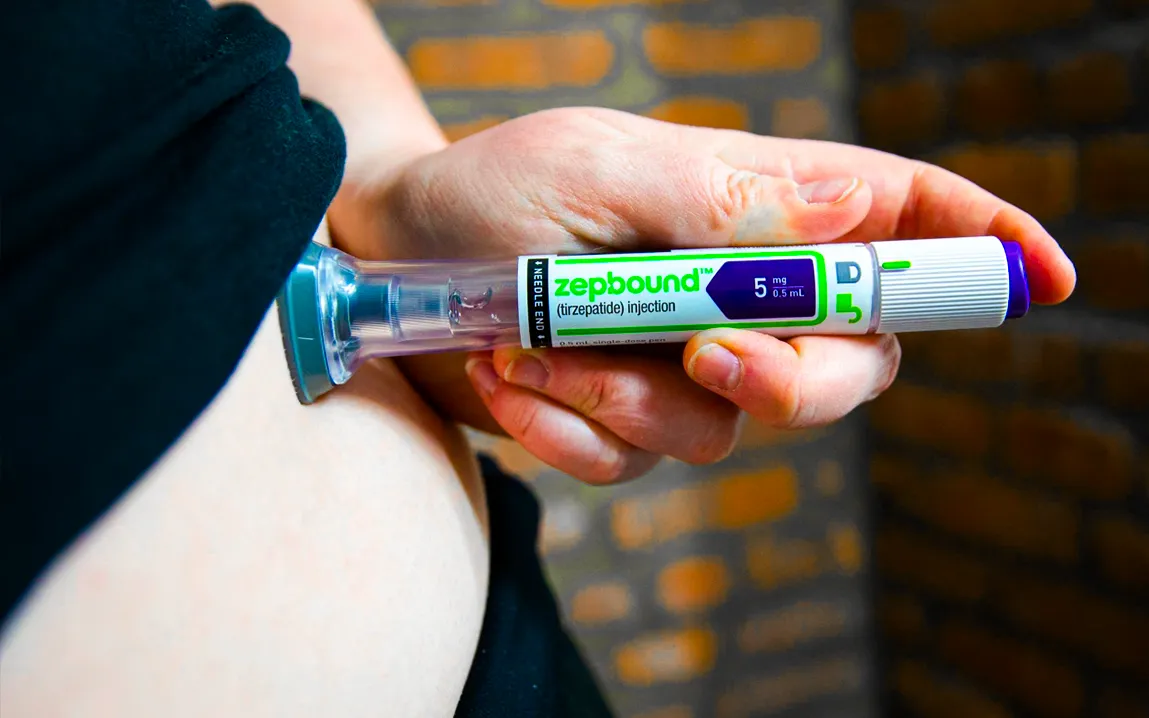
In a reversal, the FDA has reevaluated the drug’s scarcity status while permitting compounding pharmacies to continue producing tirzepatide, a crucial component of well-known medications for diabetes and weight reduction.
Patients who depend on reasonably priced compounded alternatives now have respite thanks to this judgment.
Friday, the FDA issued positive news for patients using compounded forms of tirzepatide, the active component of the diabetic and weight-loss medications Mounjaro and Zepbound.
The agency declared that until it reexamines the medication’s shortage status, compounding pharmacies would be permitted to resume producing tirzepatide.
The FDA first halted compounding pharmacies from filling new orders on October 2 after declaring the shortfall to be over.
Due to supply issues brought on by its off-label use for weight loss, tirzepatide had been on the FDA’s shortage list for almost two years.
Due to the expensive cost of the name-brand medication, patients like Simone Williams from South Carolina were obliged to use compounded alternatives. Williams stated, “The FDA’s update is good news,” but she is still wary until a final judgment is reached.
For patients who depend on these reasonably priced alternatives, the FDA’s filing provides a little reprieve. Compounded tirzepatide has helped Elizabeth Kenly, 59, lose 30 pounds. She expressed relief and said the pharmacy had been her lifeline.
Compounding pharmacies can now carry on with their operations, providing patients with comfort as they await the FDA’s ultimate ruling.