In a major step forward for energy storage technology, researchers have developed a superionic conducting electrolyte that could enhance the stability and performance of all-solid-state lithium metal batteries (ASSLMBs). This development represents a significant breakthrough in the ongoing interface issues such as dendrite formation and instability during high-capacity charging and discharging cycles that have been longstanding impediments for lithium-based capability batteries.
The Promise of All-Solid-State Lithium Metal Batteries
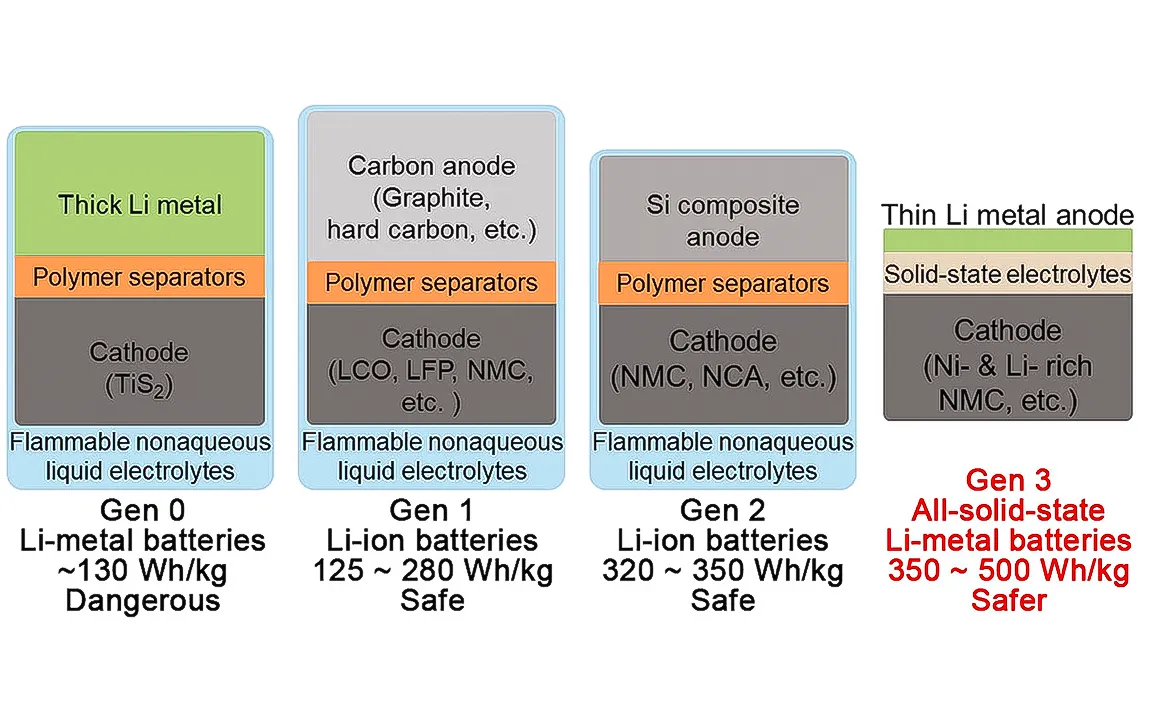
In recent years, the all-solid-state lithium-metal battery has garnered great attention for overcoming the energy density and safety limits of their earlier liquid-electrolyte counterparts, lithium-ion batteries. Solid-State batteries replace standard liquid electrolytes with solid-state electrolytes, and lithium metal anodes could become a very promising solution for energy storage contenders since they could store more energy than the conventional graphite anodes. However, hurdles like dendrite growth, low ionic conductivity at room temperature, and poor interface stability have hindered their wide implementation.
A material of the electrolyte is, in fact, one of the most important aspects of the success of ASSLMBs. There is a transport of lithium ions that the electrolyte provides, and this is generally during charging and discharging. A suitable electrolyte should be able to allow a high amount of lithium-ion conductivity and demonstrate chemical stability while precluding reactions that could damage the lithium metal and thus pose serious threats to the durability of batteries. For the longest time, it has been a challenge to find an electrolyte that can satisfy both of these conditions without sacrificing performance.
A New Superionic Conducting Electrolyte
The breakthrough is a superionic conducting, vacancy-rich β-Li₃N electrolyte. Researchers have shown that the new electrolyte indeed possesses superior properties that render it a formidable candidate for use in all-solid-state lithium metal batteries: highly lithium-compatible, stable under ambient conditions, and allowing for fast lithium-ion migration. Its superior performance is rooted in the vacancy-rich structure of β-Li₃N.
Superionic conduction is basically a phenomenon of a three-dimensional network through which rapid transportation of host lithium ions becomes possible with very high ionic conductivity. Essentially, it guarantees the highly efficient operation performance of a lithium metal battery. Among very few reported nitride-based solid electrolytes, β-Li₃N demonstrates extremely high ionic conductivity of 2.14 × 10⁻⁴ S cm at room temperature.
Overcoming Lithium Dendrites
One of the major challenges to the realization of solid-state batteries is the growth of lithium dendrites, spiny structures that can form during charging and penetrate the solid electrolyte, with possible catastrophic shorting or battery failure. These dendrites are more apt to form at high current densities and during cycles of high capacity, but the new β-Li₃N electrolyte has been shown to suppress such dendrite formation. The mechanism behind this is linked to the material’s structure, which allows for stable lithium stripping and plating even at high current densities-a key feature for long-term cycling stability.
The material also exhibits high lithium-metal compatibility, which ensures the prevention of unwanted side reactions between the lithium metal anode and the electrolyte. This is critical for ensuring the longevity and safety of the battery. What’s more, the β-Li₃N electrolyte remains stable even in air, avoiding the common problem of electrolytes degrading or reacting in moisture-laden environments that has plagued so many other solid-state electrolytes.
Improved Battery Performance
The impact of this new electrolyte on battery performance is striking. Using the β-Li₃N as an electrolyte, researchers have achieved unprecedented performance in all-solid-state lithium metal batteries. These batteries have demonstrated high energy densities, long cycle lives, and fast charging and discharging capabilities.
Most impressively, the cycle life for batteries is just fantastic. In combination with lithium cobalt oxide (LCO) and nickel-rich cathodes (NCM83), these all-solid-state batteries showed capacity retention rates of 82.05% after 5,000 cycles for LCO and 92.5% after 3,500 cycles for NCM83. This exceptional stability over a large number of charge-discharge cycles far exceeds the results from earlier prototypes of solid-state lithium metal batteries.
These batteries also realized a fast charge/discharge rate of up to 5.0 C, with a retention capacity of 60.47% even at this high speed. Therefore, this makes them potential candidates for high-demand applications such as electric vehicles, where their fast charging and high energy density are at the front line. Besides, the all-solid-state lithium metal pouch cells showed high areal capacity, further enhancing the possibility of their application in compact energy storage systems.
The Path Forward
The successful development of superionic conducting β-Li₃N electrolyte introduces a very important step towards the creation of all-solid-state lithium metal batteries. Although significant challenges persist over scaling up production and the ability of such batteries to survive long-term commercial utilization, this work opens new routes toward next-generation high-performance energy storage devices.
This, therefore, might be a much-wanted solution for the ongoing limitations of current battery technology, especially in the fields of electric vehicles and renewable energy, which are also seeing growing demand for cleaner, more efficient means of energy storage. When commercialized, these new solid-state batteries could bring greater safety, longer life, and performance that surpasses traditional lithium-ion batteries in everything from smartphones to EVs.
In the end, this means that the vacancy-rich β-Li₃N electrolyte represents an exciting leap forward in battery technology by offering a promising pathway to stable, high-performance all-solid-state lithium metal batteries. These could contribute a great deal toward the supply and storage of energy required around the world in the years to come, enabling a sustainable and efficient energy future.