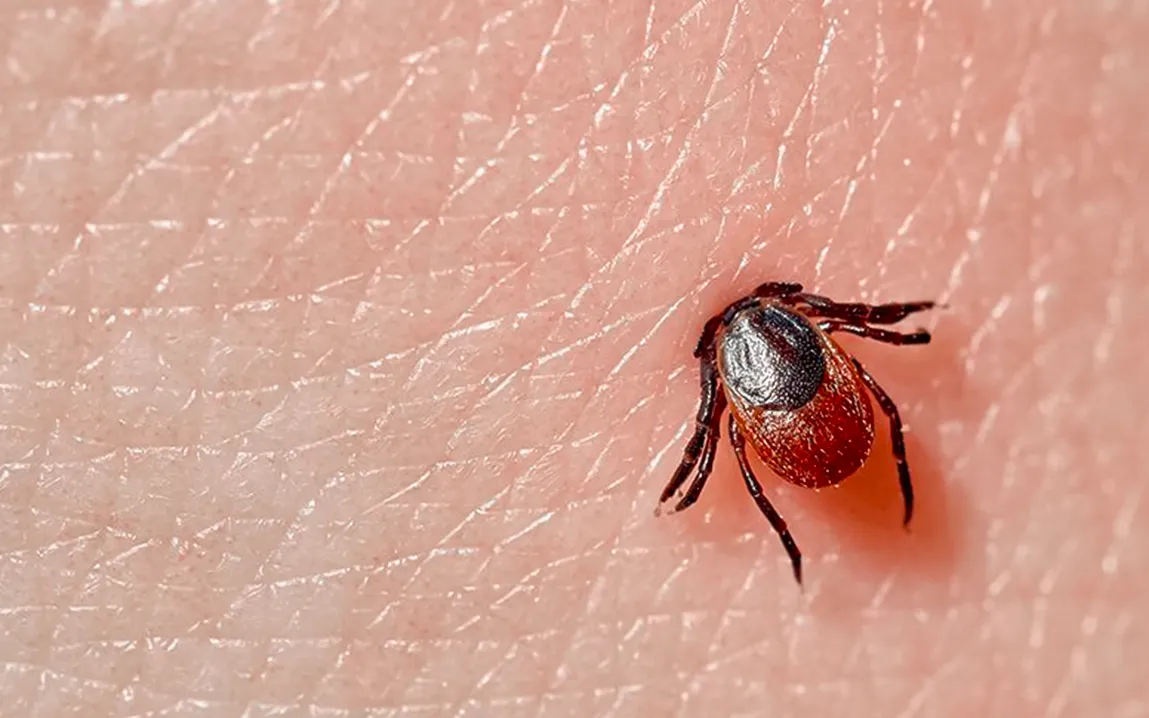
For years, ticks have been among the most feared pests due to their notorious ability to attach themselves to a host and remain there for as long as possible, thereby transmitting life-threatening diseases like Lyme disease and Rocky Mountain spotted fever. But what has remained a mystery is the exact mechanism by which the ticks manage to cling to their hosts so effectively. Recent studies have gone a long way in unraveling the underlying complicated physical chemistry of this incredible ability and have shed light on the molecular interactions that enable a tick to attach itself to skin for days at a time.
The Secret of Tick Adhesion
Ticks depend on a specific biological secretion commonly referred to as “cement” to stay attached to their hosts. This cement is produced by the salivary glands of the tick and thus forms a glue-like connection between the tick and the host’s skin. The beginning of this process is that a tick punctures the skin with its mouthparts, and in addition to feeding, it releases this cement to secure its position. The saliva of the tick is important in the modulation of the host immune response, but until recently, the chemical and physical nature of this cement was not well characterized.
Researchers at Wageningen University and Maastricht University, in collaboration with scientists from EnzyTag BV in the Netherlands, have discovered some vital information about the molecular nature of this adhesion process. Their research investigates the interactions of glycine-rich proteins, one of the major components of the cement. These proteins transition from a liquid-like to a solid-like phase, forming a bond strong enough to resist the host’s defenses, which try to remove the tick.
Phase Separation and the Development of Adhesion
This point refers to one of the impressive findings of the study that pertains to the development of phase separation in the saliva of the tick: when the saliva containing GRPs exposes itself to the skin, it starts a so-called coacervation procedure under which the proteins become segregated into different phases. This segregation causes it to form droplets packed with proteins at high densities and aggregate to form the viscous substance. The resulting adhesive is highly effective at binding to the skin, anchoring the tick in place for the duration of its feeding session.
This phase separation depends on various environmental factors, such as the salt content of the host’s skin and the evaporation of moisture from the tick’s saliva. By using microfluidic experiments together with high-resolution imaging, this team of researchers has investigated this phase transition and visualized how the proteins self-organize into condensates that show a high degree of adhesion. It suggests that upon contact with skin, the saliva of the tick changes dynamically in character to form an improved bond with the host.
Molecular Interactions Behind the Stickiness
This is further investigated down to the level of specific molecular interactions responsible for the cement being so sticky. It was observed that certain amino acids within the GRPs, mainly arginine and aromatic residues such as phenylalanine and tyrosine, were important in the adhesion process. These amino acids form cation-pi and pi-pi interactions that greatly increase the stickiness of the tick’s saliva. These interactions are not just a random occurrence but a finely tuned system that has evolved to maximize the effectiveness of tick attachment.
The research team also observed that over time, as the tick feeds, the adhesive properties of the cement become stronger. Initially, the saliva forms a liquid-like layer, but as the tick remains attached, this material gradually solidifies. This aging process strengthens the bond so that the tick cannot be dislodged easily even when the host tries to remove it. This, in turn, is quite an effective mechanism for ticks to feed over a period of days without getting removed by the host.
Implications for Tick-Borne Disease Prevention
The physical chemistry of understanding the adhesion of ticks holds an important place in designing newer strategies against tick-borne diseases. Identification of crucial molecular components in tick attachment provides a lead in making specific interventions. In designing new treatments, for example, disrupting the ability of a tick to produce or release such adhesives could potentially not allow it to attach itself in the first instance.
In addition, such knowledge from this study might be useful in creating innovations in biomaterial science. The cement of the tick has some unique adhesiveness that could be used in developing new kinds of medical adhesives. Such adhesives would have the advantage of being strong, flexible, and able to bond to sensitive tissues without causing any damage. This may be applied in wound care, where a strong yet gentle adhesive is needed.
Conclusion
The investigation into tick adhesion has unraveled an intriguing interaction between biological and physical chemistry in which these pests are capable of holding onto their host for extended periods. By investigating the molecular mechanisms of tick attachment, researchers have not only advanced our understanding of the biology of these ectoparasites but have also made it possible to develop a new generation of methods in the prevention of diseases transmitted by ticks. With the world confronted with an increasing problem of diseases caused by ticks, this study represents a promising step toward the elaboration of more effective methods of defense against these persistent and hazardous vectors for human and animal populations.